Print Technology in Biotechnology
The working group ‘Print Technology in Biotechnology‘ is working on all aspects of the bioprinting process development.
A prerequisite for a successful print is the development of tailor-made bioinks whose formulation includes network-building and stabilizing additives. These are the matrix for the biologically active components, for example enzymes or eucaryotic cells. During the bioprinting process, functional hybrid objects for the use in pharmaceutical and medical research are created.
For the industrial application, the bioprinting process should be reproducible and robust. On that account, optimized devices and processing protocols are developed aiming to control the process steps during and after printing for SLA, inkjet and extrusion-based 3D-printing.
In parallel with process control, quality control strategies are being established. Examples are rheological characterization of the used bioinks, in-process-control methods as well as the characterization of the 3D-printed object. This includes the measurement of the metabolic activity of incorporated cells. Among the techniques used are the automatized image analysis, mechanical tests as well as various assays.
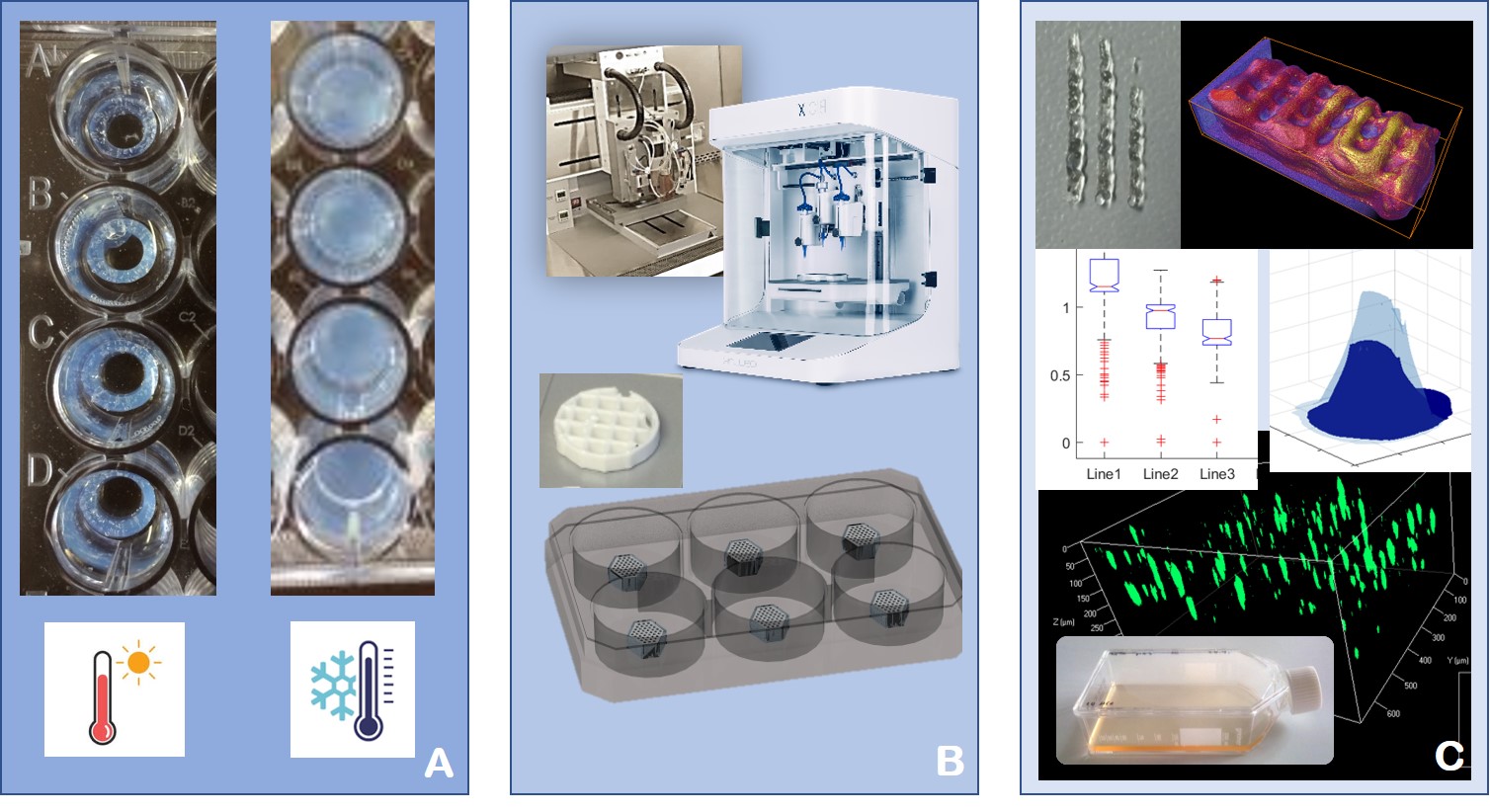
Figure 1 Process steps in Bioprinting. A: Influencing the degradability of the bioink by additives, for example with Kolliphor®, a thermoresponsive additive that melts at low temperatures. B: Process development using several bioprinting systems. For HT applications, the printed objects have to be produced with high reproducibility and robustness. C: Characterization of bioprinted objects by several techniques.
Thesis
If you are interested in a Bachelor's or Master's thesis in this subject area, please contact Svenja Strauß or David Grijalva Garces by e-mail.
