Layered Double Hydroxides – Nano-containers
Layered double hydroxides (LDH) are build of positively charged layers. Anions together with water are incorporated into the interlayer to compensate the charge. The positively charged layers consist of a hexagonal closed packed OH-groups in which the octahedral positions are occupied by differently charged cations. Due to the structural similarity to layer silicates (cationic clays, surplus of negative charge) LDHs are often named anionic clays.
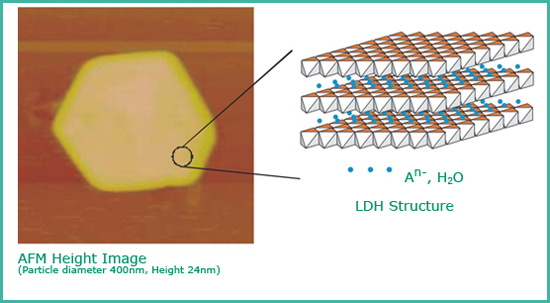
Applications of LDHs are manifold. e.g. anionic exchangers, adsorbents or catalysis, just to name a few.
LDH exits naturally as Hydrotalcite (Mg6Al2(OH)16[CO3]∙4H2O) but are easily synthesized. The synthesis variables are pH-value, temperature, and especially the type of anion and the cations, as well as the ratio of the metal-ions.
Being a anion intercalating agent, the LDH renders an ideal nano-sized carrier for drugs. The LDH exhibit a 2-3 fold exchange capacity than layer silicates, their structural features can be mass tailored.
